Private CBD maker British Cannabis has jumped on top in the UK’s CBD sweepstakes as the Food Standards Agency (FSA) again doubled the total number of products that still have a chance for full authorization.
FSA has now finalized a list of 11,908 products that have passed the first stage of the agency’s three-stage review. That is up from a total of 6,000 products approved by FSA, as per a previous update published in late April.
Jumping ahead
British Cannabis (registered as CBD Health Food Ltd.), Berkshire, saw its number of products nearly quintuple from the 310 that made FSA’s original list released on March 31, reaching a total of 1,471.
RX Pharmatech Ltd., Bradford, West Yorkshire, which has ties to U.S. cannabis interests, slipped to second place on the updated list, with 801 products, more than double the number previously approved.
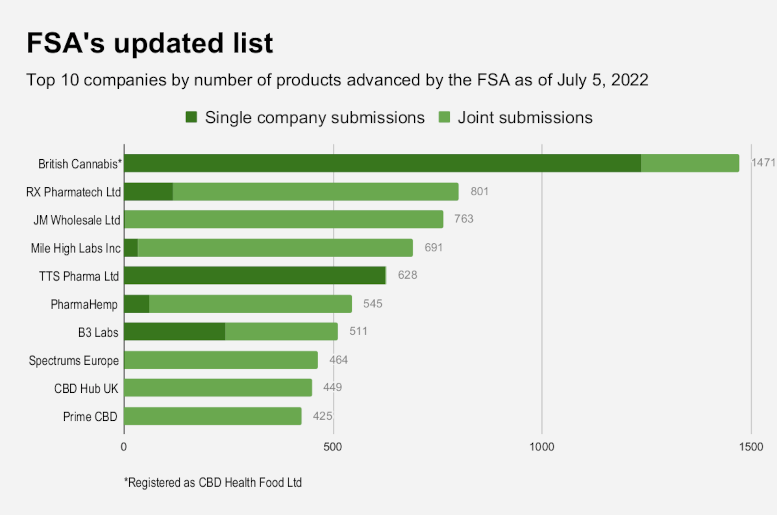
Both companies’ totals represent products submitted independently and in tandem with other producers via joint applications.
Products on the FSA list may remain on sale as their food safety applications continue through the agency’s approval process for new or “novel” foods. Any that failed to make the list must be removed from the market. To qualify, products had to have been in distribution on what was essentially a gray market before Feb. 13, 2020. Those introduced after that date were not eligible for FSA’s consideration.
Again, and again
The updated list marks the second time the FSA has let more products enter the approval process after producers earlier complained about the agency’s review system. The complaints came upon the release of an original list of 3,536 products under an application deadline of March 31.
Citing clerical errors, FSA added nearly 3,000 products in late April amid the complaints and doubts about the credibility of the certification regime. The agency then reopened the application process under a deadline further extended to May 26. FSA now says the list is closed.
“The only changes we envisage being made to the list now will be to reflect the status of (already listed) products as we move to authorization, and any routine corrections,” said Rebecca Sudworth, FSA Director of Policy.
Elimination
FSA has stressed that the listed products could still be eliminated. Approvals granted so far are only for the pre-validation or “awaiting evidence” stage. The products must now pass through “risk assessment,” the second stage, for which producers must provide toxicological and other studies, before moving on to await FSA’s decision on full authorization, the final stage.
In the meantime, FSA is urging caution on the part of the buying public. “Consumers should continue to think carefully before consuming CBD products because we don’t know a lot about them,” FSA said in a statement, suggesting women who are pregnant, breastfeeding or taking medication should not consume CBD products. Otherwise, FSA urged consumers to take no more than 70mg of CBD per day (about 28 drops of 5% CBD) unless a doctor recommends otherwise.
Eligibility
The FSA process is intended to clarify a flourishing gray market that has seen thousands of CBD products show up in retail outlets over the past several years. To qualify, products already in distribution had to have been on the market before Feb. 13, 2020. Those introduced to the market after that date were not eligible for the agency’s consideration.
There are no fully approved CBD products on sale in the UK at present, but a flourishing gray market has seen thousands of products show up in retail outlets over the past several years. The Association for the Cannabinoid Industry estimated the domestic CBD market was roughly £690 million ($929 million/€824 million) in 2021.
A year of waiting
FSA said it expects the first CBD products to be authorized during the latter half of 2023, “However, this will be dependent on the availability of evidence that allows food safety assessments to be completed. The authorization process can take more time, especially where additional studies and evidence are required to complete the process.”
CBD companies are now free to apply for authorization of new-to-market CBD food products, which must pass through the FSA’s standard review, the agency said.
Businesses wanting to sell their products in England and Wales must apply through FSA. CBD products for sale in Scotland are subject to a separate authorization process managed by Food Standards Scotland while Northern Ireland producers come under EU rules as a result of Brexit protocols.

