More than 400 CBD products have now been stricken from a public list by UK food safety officials, and may not be sold to consumers.
The UK’s Food Standards Agency (FSA) this week further trimmed the number of products under consideration for legal market access under rules for new or “novel” foods, and now has eliminated a total of 409 products since the agency’s review started last year.
More than 11,000 products remain on the agency’s list after having passed a pre-validation (or “awaiting evidence”) stage, a preliminary review in the novel food approval process. FSA began eliminating products in March 2022. In the biggest batch stricken last year, some 100 products were removed in September. Another 300 or so fell off the list when it was updated Monday.
132 products ‘validated’
Meanwhile, FSA has now moved 132 products into validation (or “risk assessment”), the second stage of the novel foods review for CBD, which involves toxicological and other studies, and can lead to full market authorization, the final stage. Two U.S.-based producers, cbdMD, Charlotte, North Carolina, and Charlotte’s Web, Denver Colorado, lead the “validated” list in terms of product numbers.
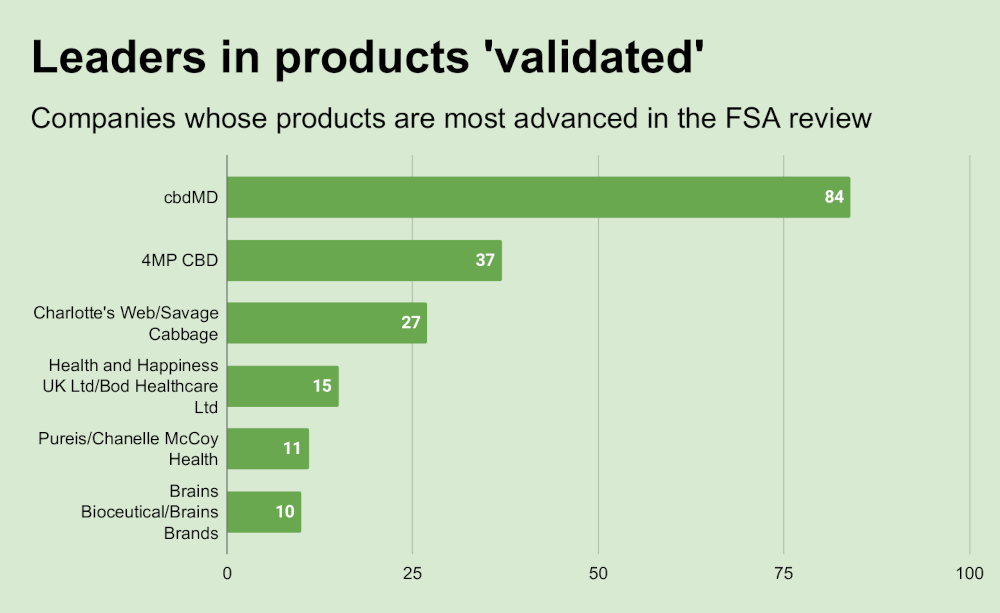
FSA’s novel foods unit is addressing a CBD gray market that has flourished in the UK, leaving consumers at risk. The agency’s review is a one-time opportunity for CBD makers currently on the market to get their products fully legal. Products struck from the list may no longer be offered to consumers under FSA rules.
More than 300 product applications that have been removed from the FSA list were from JM Wholesale Ltd. of the UK. But that company still has more than 400 products that continue in the first stage of the review. British Cannabis, which submitted the most products to the FSA (1,471) review process, has seen 15 products disqualified; and RX Pharmatech, the No. 2 applicant (801), had eight fall off the list but still has nearly 800 in contention.
FSA struggles
FSA, whose review of CBD has been troubled from the start, does not give reasons for the disqualification of specific products, but some stakeholders have complained that many were not on the market as of Feb. 13, 2020, a preliminary requirement in the review process.
But companies were required to submit only receipts, contracts or invoices showing their products were in circulation before the February 2020 cutoff, and the FSA has no mechanism in place to verify that documentation. Critics have said it’s highly unlikely that all of the 12,000+ products that originally passed the preliminary stage of the FSA review met that requirement. That may be the reason for elimination in some cases: Some products may have never been on the market at all.
Products that remain on the public list may stay on the market until further decisions based on toxicology and other technical benchmarks, phase 2 in the approval process, if they are not de-listed before they get there.
FSA said the elimination of products from the public list can also be due to duplication, at the request of the applicant, or other reasons which make them non-compliant under UK food law.
Licking their chops
UK and international CBD makers, struggling amid a lingering crash in the CBD sector, are anxious to get into the UK market, which some analysts have said is now the second biggest in the world; the market is projected to reach $1 billion over the next few years.
But the approval process under the FSA has been extremely slow to unfold, and is likely to take two years or more at the current pace of review.
FSA was inundated with CBD applications early last year after stakeholders complained about the review process that guides the one-time chance for producers to get their gray-market products legal. The agency eventually reopened the application window by one full year, which eventually brought a flood of products to the list, nearly quadrupling the original number under review from roughly 3,500 to more than 12,000.
Companies that wish to enter the UK CBD market that did not qualify under initial rules may submit regular novel food approval applications directly through the agency.

