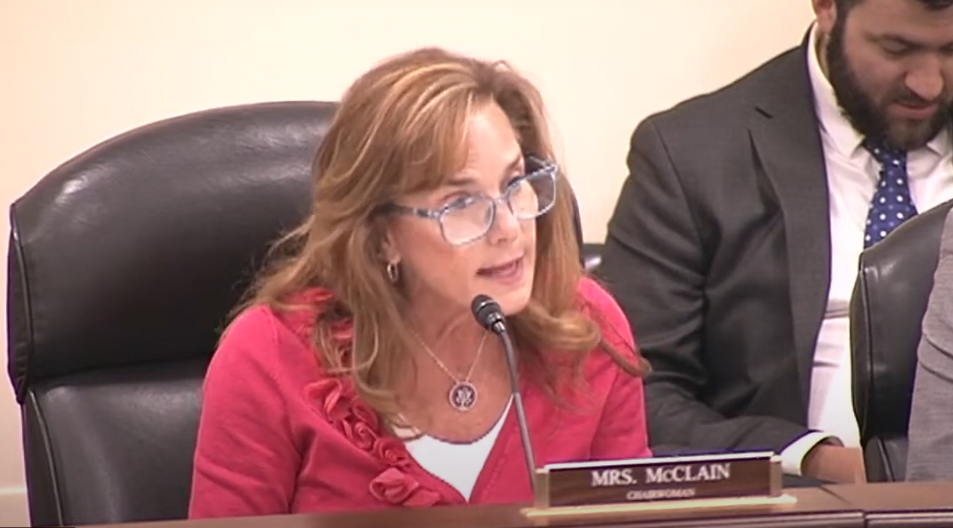
“Hemp in the Modern World: The Years Long Wait for FDA Action,” this week’s congressional hearing in the U.S. Congress, will do little to change the arc of things for the CBD sector.
The hearing, which ran to roughly 90 minutes yesterday, served mostly as an opportunity for lawmakers to bellow, however softly, in the manner typical of politicians these days. “Softly” because hemp, in its short modern-era history, has always been a bipartisan issue.
Flashpoints
But because there must be disagreement, the Republicans, who chaired the hearing, criticized the FDA and said the agency needs to get on with setting rules for CBD under existing legislation and regulations, to eliminate risks to consumers.
Democrats agreed that the unregulated CBD gray market poses safety issues but sided with the Biden Administration’s FDA, which has called on Congress to set a general framework for the products through a new law on which it can base specific safety rules – resulting in a “new path” for the category.
Legislation is already drafted to do just that, most recently through the Hemp Access and Consumer Safety Act. An existing bill recently re-introduced, the Act would update federal standards to authorize that hemp-derived CBD products are regulated by the FDA as dietary supplements, foods and beverages under the federal Food, Drug and Cosmetic Act (FD&C Act). It’s what everyone seems to want, and is backed by both Republican and Democrat lawmakers.
Blame all around
In truth, FDA failed to act during both Trump and Biden administrations before the agency finally declared in January that existing federal safety standards are insufficient to manage the CBD sector.
For now, FDA recognizes CBD as a drug, technically barring it from use in foods or being marketed as a dietary supplement. But FDA has already released a set of non-binding recommendations for CBD, and said once Congress passes a law that it can establish new rules rather quickly – a relative term in the notoriously slow-moving FDA, but nonetheless indicating some initiative at the agency.
Democrats and Republicans also sparred over CBD research during this week’s hearing, with Republicans suggesting existing research is sufficient to ensure consumer safety and Democrats hewing to the FDA position that long-term research, by its very nature, is still lacking for CBD, which has been around only for about five years.
The FDA has repeatedly raised concerns regarding CBD’s potentially harmful effects on pregnant women and fetuses, young children, the elderly, and the liver and male reproductive system. FDA released a paper in March based on existing clinical studies which concluded that long-term consumption of CBD needs further study.
Congress at fault
It was Congress itself that failed to anticipate CBD and other hemp-derived cannabinoids in hemp in the 2018 Farm Bill, although CBD products had already been on the market for about two years. Furthermore, who in Congress or the hemp industry could have predicted the unthinkable – that hemp would hit the market in the form of high-producing delta-8 THC, a synthetic “marijuana” made from CBD – after hemp stakeholders had worked hard to separate hemp from weed in the public’s mind. (Delta-8, the hemp product that presents the biggest health risk, was barely mentioned in Thursday’s hearing.)
Perhaps the most cynical comments from yesterday’s hearing came from stakeholder representatives. They joined some lawmakers in blaming CBD’s current woes on a lack of rules from the FDA when we all know that it was the hype, the hustlers and the hucksters, the creators of an unbridled gray market in the first place who eventually brought the sector to its knees.
Through it all, “Hemp in the Modern World: The Years Long Wait for FDA Action” brought absolutely nothing new to the debate over CBD. The die seems to be cast here. Laws are proposed, FDA says it’s ready to move forthrightly on rules once the legislation is in place. Yesterday’s gabfest, in the end, doesn’t change anything.
What they said
Highlights from comments made during the Congressional hearing “Hemp in the Modern World: The Years Long Wait for FDA Action”:
Rep. James Comer, Republican of Kentucky: “Even though we have more and more data available to regulators to make appropriate decisions about CBD in the marketplace, the FDA has taken no meaningful action to provide clear guidance and certainty in the market, refusing to regulate CBD products under existing lawful pathways. Without FDA regulations, the good faith producers of these products are left with no path forward and consumers are left in the dark.”
Rep. Jamin Raskin, Democrat of Maryland: “We need reasonable regulation of the hemp and hemp derivative marketplace to protect consumers and to ensure the good actors in the hemp industry can grow their businesses and we could have a legitimate and flourishing market in hemp. But we need to make sure that the regulations make sense. FDA realistically cannot regulate the entire world of hemp and its derivatives without additional research authority and resources.”
Rep. Lisa McClain, Republican of Michigan: “FDA announced earlier this year that it’s needs a new regulatory framework for hemp and CBD. Translation: ‘Give us more authority. Give us more money. Give us more staff and only then will we actually do our duties under the law.’ This announcement has led to confusion and uncertainty in the market, which has suppressed the ability for good faith manufacturers to sell CBD products. It only benefits bad actors who capitalize on the confusion and the flood of the market with potentially unsafe products. The FDA must do better and use their already existing authority to regulate how derived products you know actually do the job they were signed up to do.”
Rep. Katie Porter, Democrat of California: “The problem is that some of the same lawmakers who want to do oversight of the FDA for being cautious about its existing powers would turn around and blast the agency if they ever felt like it went too far beyond its legal authority. Look, we can’t have it both ways here. The FDA knows Congress will appropriately hold it accountable if the agency exceeds its authority. That’s our job. So the FDA is not going to take the risk of going too far.”
Jonathan Miller, general counsel, U.S. Hemp Roundtable: “Unfortunately, the U.S. hemp industry has been struggling considerably in the last few years. And this turmoil is due in large part to decisions made by the FDA. The hemp industry may be unique in that we are coming to Congress to ask: Please, regulate us. Regulatory relief for the hemp-derived CBD industry constitutes an economic stimulus package for the nation’s farmers and small businesses without requiring one dime from the American taxpayer.”
Gillian Schauer, executive director, Cannabis Regulators Association: “Whether through the Farm Bill or another priority piece of legislation, a broad regulatory framework is urgently needed to address hemp-derived cannabinoid products. Congress has an opportunity to learn from the approaches that states have taken to set a thoughtful and comprehensive federal regulatory framework. The regulation of hemp-derived products is complex and nuanced, and state regulators understand those nuances better than anyone.”