[This is the third in a series of stories looking at the CBD certification process in the UK]
An additional 2,445 CBD products have been preliminarily approved by the UK Food Standards Agency (FSA), bringing the total number of products that still have a chance for full authorization to roughly 6,000.
FSA added the products yesterday in response to an uproar over its approval system that followed the March 31 release of an original list of 3,536 products that advanced in the agency’s system for certifying new or “novel” foods.
FSA last week reopened the window for companies to apply for authorization of CBD products amid questions over the credibility of the certification process. Those still absent from the updated list still have until May 26 to submit applications. The original deadline for applications was more than one year ago, March 31, 2021.
170 new companies appear
Roughly 170 newly listed companies are associated with the 2,445 products added this week. Some companies submitted products independently while others were part of joint applications by multiple companies; most were associated with products preliminarily approved and placed on the FSA’s original March 31, 2022 list, according to FSA.
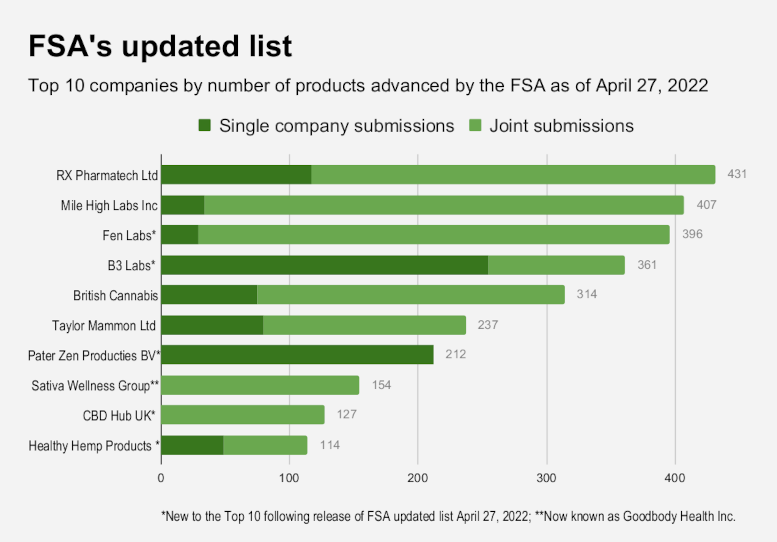
“Over 1,700 new products added to the list relate to applications which had been submitted to the FSA prior to the 31 March (2021) deadline, but which required further evidence from businesses to ensure the products met the public list criteria before they could be added,” FSA said in a press statement. “Around 700 products have been added to correct a clerical error,” the agency added.
Eight of 23 products that have been sold at major retailers but which failed to make the original FSA list released last month have now been added as a result of yesterday’s update. They are: Body and Mind, CBDfx, Drink 420, Holistic Herb, KIKI, Simplee, The Leaf Life and Vita Coco.
Those products, others added via the recent update, and any accepted between now and May 26 that meet other FSA criteria will be allowed to remain on the market pending the progress of their applications, FSA said.
Running total: 5,981 products
The FSA process is intended to clarify a flourishing gray market that has seen thousands of CBD products show up in retail outlets over the past several years. To qualify, products already in distribution had to have been on the market before Feb. 13, 2020 under FSA guidelines. Those introduced to the market after that date were not eligible for the agency’s consideration.
In total, 543 companies are associated with 5,981 products on the FSA’s list, according to the update this week. All but a handful of those companies are listed as “awaiting evidence” at the second stage of the agency’s three-stage approval process and may remain on sale as their applications continue in the FSA system.
EIHA’s numbers rise
EIHA Projects GmbH, a consortium of CBD companies organized by the European Industrial Hemp Association, saw its total number of products under two applications, for isolates and full-spectrum products, rise from 1,992 to 3,194 with the release of the updated FSA list – or roughly 53% of all products that have now received preliminary approval.
A second consortium under the Association for the Cannabinoid Industry (ACI) said its 18 members accounted for 2,106 products — about 35% — that are on the list.
“We are urging any CBD businesses with evidence they have that links their products to credible applications to send it to us as soon as possible, but no later than 26 May 2022 for consideration. We will not be accepting evidence for products to be included on the public list after this date,” Rebecca Sudworth, Director of Policy, FSA said
The agency has indicated it will continue to update the public list through June 30.
Nothing is final
FSA has stressed that approvals granted so far are preliminary and that some products could be eliminated as they move through the agency’s review. Businesses whose products are preliminarily approved are required in the next stage of the certification process to provide toxicological and other studies. Evidence that those reports have been commissioned must also be submitted before May 26.
The UK’s market for CBD products was estimated to be worth £690 million (~€814.5 million; ~$905.7 million) in 2021, according to ACI, which has said that makes the UK the world’s second-biggest consumer market for CBD in the world behind the USA.

